Researchers at Scripps Research Institute have used patient-derived stem cells to create brain organoids—also called “mini-brains”—to investigate a rare and severe form of autism spectrum disorder (ASD) linked to intellectual disability. These models provided insights into how a specific genetic mutation disrupts brain development and allowed the team to test an experimental drug, NitroSynapsin, which reversed some of the identified dysfunctions.
The study, published in Molecular Psychiatry, sheds light on the molecular effects of mutations in the MEF2C gene, a key regulator in brain development. It also suggests that targeting imbalances caused by such mutations later in life could mitigate some ASD symptoms, offering hope for future therapies.
ASD is a complex neurodevelopmental condition characterized by challenges with social interaction, communication, and repetitive or restricted behaviors. It varies widely in severity and can be accompanied by intellectual disabilities, sensory sensitivities, and medical issues such as epilepsy. Despite extensive research, the exact causes of autism remain elusive.
Genetic mutations are thought to play a significant role, with hundreds of genes implicated. However, the molecular mechanisms that connect these genetic changes to autism’s behavioral and neurological symptoms are poorly understood, creating barriers to developing effective treatments.
One specific genetic condition associated with autism is MEF2C haploinsufficiency syndrome. This rare disorder results from a mutation in one copy of the MEF2C gene, which disrupts its ability to produce sufficient levels of a protein critical for brain development and function. Individuals with MEF2C haploinsufficiency often experience severe developmental delays, limited or absent speech, stereotypic movements, and frequent seizures.
The MEF2C gene is a key regulator of other genes that influence brain cell development and synaptic function, making it an important area of study. However, how exactly this mutation leads to the severe symptoms seen in patients remained unclear, limiting the ability to design targeted therapies.
The motivation behind the study was to bridge this knowledge gap and explore whether the disruptions caused by MEF2C mutations could be mitigated or reversed. By studying patient-derived brain models, the researchers aimed to better understand how the mutation affects the development and function of neural circuits. Additionally, the researchers sought to evaluate the therapeutic potential of NitroSynapsin, an experimental drug, to determine whether it could address the neural dysfunctions caused by MEF2C mutations.
“Our group was the first to discover and clone the transcription factor named MEF2C some years ago, but more recently we and others recognized its importance not only in development, maintenance and aging resilience in the nervous system, but also to the development of a severe form of ASD and developmental intellectual disability in individuals bearing certain mutation in one copy of the gene encoding MEF2C (termed MEF2C haploinsufficiency),” said Stuart A. Lipton, the Step Family Foundation Endowed Professor and co-director of the Neurodegeneration New Medicines Center at Scripps Research, a clinical neurologist, and senior author of the new research.
“Importantly, MEF2C also directs the expression of many other genes involved in ASD, so finding a treatment or cure for MEF2C haploinsufficiency might also help these other children. Given the very high prevalence of ASD now, affecting approximately 1 in every 36 children, this could prove very important.”
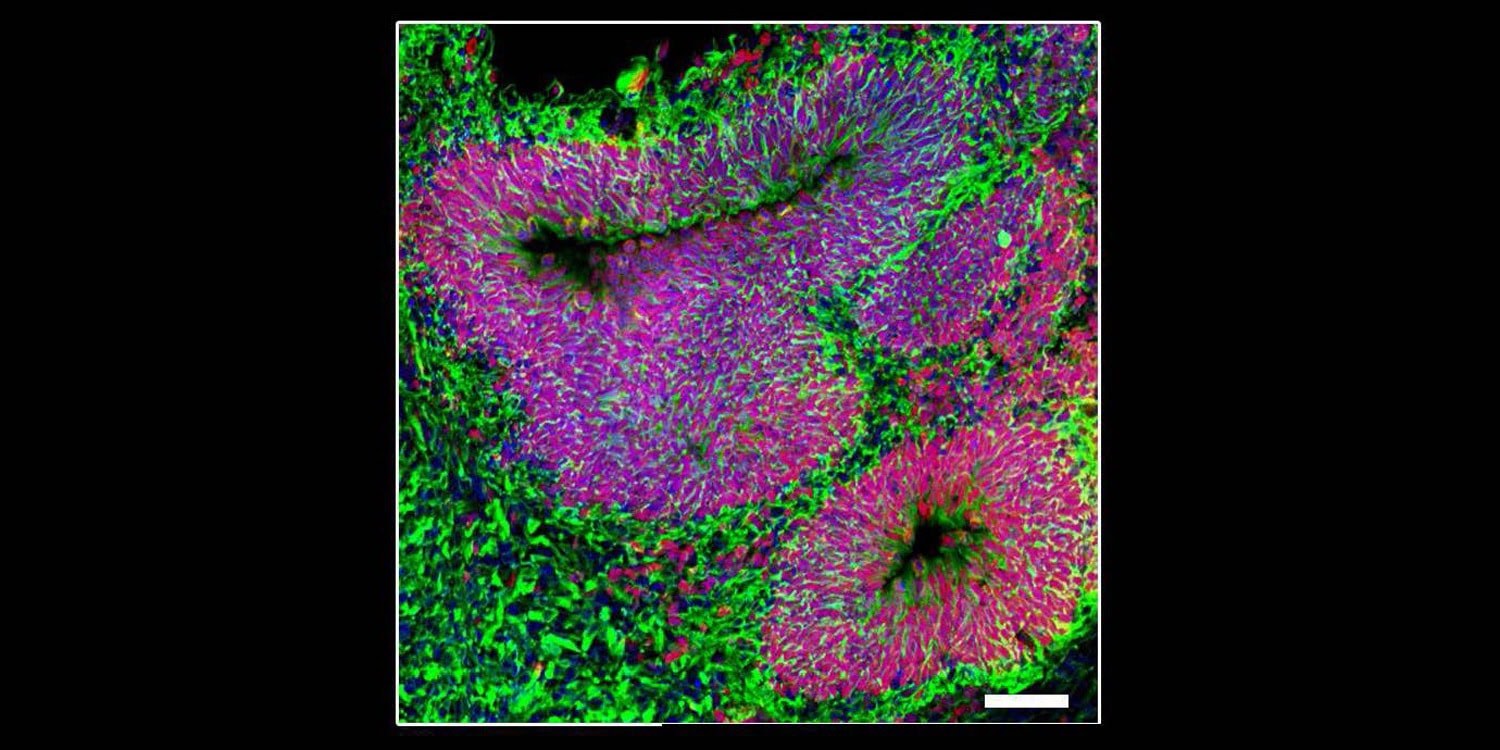
To understand how mutations in the MEF2C gene lead to severe autism symptoms, the researchers used cells from patients with MEF2C haploinsufficiency syndrome. They transformed these cells into induced pluripotent stem cells, which can develop into any cell type, including brain cells. Using these stem cells, they created lab-grown “mini-brains” (organoids) and 2D cell cultures that mimic human brain development.
“We could reproduce essential aspects of the brains of patients to study their electrical activity and other properties,” Lipton said. “We actually brought kids into the lab to see their own mini-brains and that was quite emotional for the children and families alike.”
These models allowed the team to observe how the mutation affected brain cell growth and function. They also compared the patient-derived cells to genetically edited “control” cells without the MEF2C mutation, ensuring a precise understanding of the mutation’s effects. To test potential treatments, they exposed the mini-brains to NitroSynapsin, a drug designed to regulate brain cell communication.
The researchers found that the MEF2C mutation caused an imbalance in brain cell development. Normally, a balanced mix of neurons (nerve cells) and astrocytes (support cells) is crucial for proper brain function. However, the patient-derived mini-brains produced fewer neurons and more astrocytes, disrupting this balance. This imbalance hindered the formation of healthy neural circuits, a foundational aspect of brain development.
The neurons that did form displayed hyperactive behavior. Electrophysiological recordings showed that these neurons fired excessively and out of sync with one another, mimicking the neural overactivity associated with seizures in patients. At the molecular level, the mutation disrupted the expression of genes essential for neural communication and synaptic function. One key discovery was an increase in excitatory signaling and a decrease in inhibitory signaling, creating an imbalance that could explain many of the symptoms observed in MEF2C haploinsufficiency syndrome.
The researchers discovered that MEF2C mutations disrupted the expression of specific microRNAs—small molecules that help regulate gene activity—important for brain development. In patient-derived cells, the levels of microRNAs such as miR-4273 and miR-663 were significantly reduced.
“In our study, a few specific miRNAs appear to be important in telling developing brain cells whether to become glial cells, excitatory neurons, or inhibitory neurons,” Lipton said. “Mutations in MEFC2 alter the expression of these miRNAs which, in turn, prevent the developing brain from making proper nerve cells and proper connections or synapses between nerve cells.”
When the researchers applied NitroSynapsin to the mini-brains, they found that it helped restore balance in neural activity. The drug reduced the excessive firing of neurons and corrected the imbalance between excitatory and inhibitory signals. These changes brought the activity of patient-derived mini-brains closer to that of the control models. This finding suggests that NitroSynapsin might hold therapeutic potential for addressing neural dysfunctions caused by MEF2C mutations.
Lipton was surprised by “the fact that correcting excitatory/inhibitory imbalance of electrical signals in human mini-brains, made from stem cells of patients with this form of ASD, could have such a large effect on phenotypes associated with the condition.”
However, the organoids, while advanced, cannot fully replicate the complexity of a human brain or its environment. Additionally, the findings are specific to MEF2C mutations and may not generalize to other forms of autism. Further research is needed to confirm these results.
“We have now developed our new drugs in mouse models and using human cerebral organoids of ‘mini-brains’ but real human trials are needed to test the new drugs,” Lipton said. “We are raising funds for this right now.” The long-term goal is to “complete a human clinical trial testing our new lead drug to improve the lives of children with ASD.”
The study, “Dysregulation of miRNA expression and excitation in MEF2C autism patient hiPSC-neurons and cerebral organoids,” was authored by Dorit Trudler, Swagata Ghatak, Michael Bula, James Parker, Maria Talantova, Melissa Luevanos, Sergio Labra, Titas Grabauskas, Sarah Moore Noveral, Mayu Teranaka, Emily Schahrer, Nima Dolatabadi, Clare Bakker, Kevin Lopez, Abdullah Sultan, Parth Patel, Agnes Chan, Yongwook Choi, Riki Kawaguchi, Pawel Stankiewicz, Ivan Garcia-Bassets, Piotr Kozbial, Michael G. Rosenfeld, Nobuki Nakanishi, Daniel H. Geschwind, Shing Fai Chan, Wei Lin, Nicholas J. Schork, Rajesh Ambasudhan, and Stuart A. Lipton.