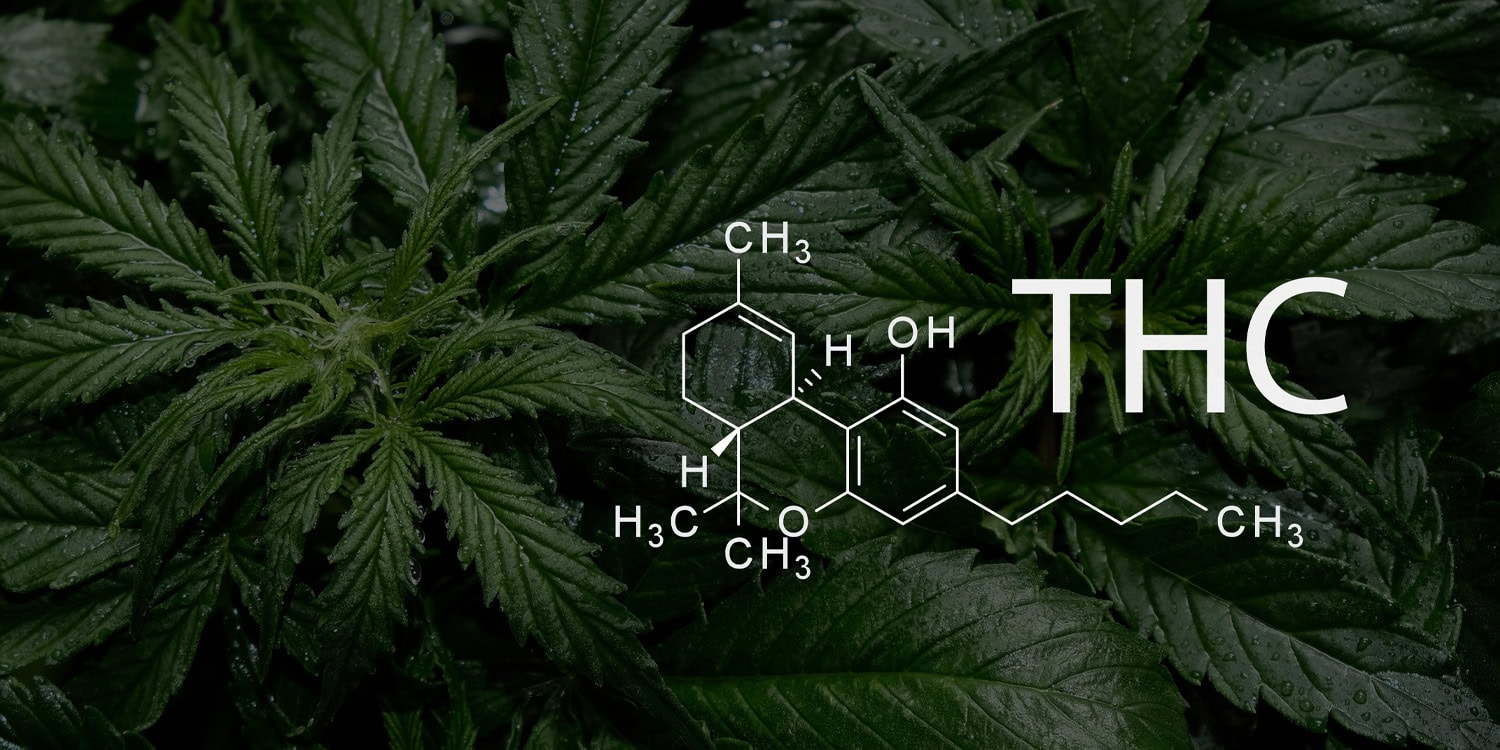
In a new study that could pave the way for new treatments targeting age-related cognitive decline, researchers have discovered that low-dose, long-term administration of a key cannabis component can reverse aging processes in the brain and has an anti-aging effect overall. The study, conducted by teams from the University Hospital Bonn (UKB) and the University of Bonn in collaboration with Hebrew University in Israel, focused on the effects of tetrahydrocannabinol (THC), the primary psychoactive component of cannabis, on aging mice.
The results, published in the journal ACS Pharmacology & Translation Science, suggest that THC can rejuvenate cognitive function in older mice by influencing key molecular pathways in the brain.
Aging is often associated with a decline in cognitive abilities, which is thought to result from the deterioration of brain cells and the connections between them. Previous research had hinted that the endocannabinoid system — a complex network of receptors and signaling molecules in the brain and other organs — plays a crucial role in this process.
Specifically, the cannabinoid receptor type-1 (CB1), which is abundant in the brain, appears to be linked to brain aging. Loss of CB1 activity in mice has been shown to lead to significant age-related deficits in learning, memory, and neuron survival.
With this in mind, the researchers set out to explore whether enhancing CB1 activity with low doses of THC could have the opposite effect, potentially reversing some aspects of brain aging. They were particularly interested in how THC might affect mTOR, a protein that acts as a central regulator of cell growth, metabolism, and aging. mTOR signaling has been linked to both cognitive performance and the aging process, making it a key target for interventions aimed at extending healthy lifespan.
To investigate, the researchers used a group of male mice that were either young (four months old) or old (18 months old). The mice were randomly assigned to receive either a low dose of THC or a placebo for a period of 28 days. THC was administered continuously through subcutaneous minipumps, which allowed the researchers to control the dosage and ensure consistent delivery.
The study focused on several key areas: brain function, the levels of specific proteins involved in synaptic signaling, and the overall metabolic state of the mice. The researchers monitored the mice’s body weight, food intake, and activity levels throughout the experiment. Additionally, they performed detailed biochemical analyses of the mice’s brains, blood plasma, and adipose (fat) tissue to assess how THC affected mTOR signaling and the metabolome—a comprehensive snapshot of all the metabolites, or small molecules, present in the body.
In the brains of the older mice, THC treatment led to a temporary but significant increase in mTOR activity, particularly in the hippocampus, a region critical for learning and memory. This increase in mTOR activity was accompanied by a rise in the levels of key synaptic proteins, such as synaptophysin and PSD-95, which are essential for the formation and maintenance of synapses—the connections between neurons.
Furthermore, the researchers observed that THC treatment significantly boosted the metabolic activity in the hippocampus. This was evidenced by increased levels of metabolites involved in energy production, such as those associated with glycolysis and the citric acid cycle, which are pathways that generate the energy needed for cellular processes. Interestingly, these changes were most pronounced after 14 days of treatment and tended to normalize by day 28.
In contrast to the brain, the adipose tissue of THC-treated mice showed a decrease in mTOR activity and a reduction in the levels of amino acids and carbohydrate metabolites, similar to what is observed during caloric restriction or intense physical exercise—both of which are known to have anti-aging effects. This reduction was particularly evident after 28 days of treatment, suggesting a dual-phase effect of THC: an initial increase in brain activity followed by a systemic shift towards energy conservation and reduced metabolic activity.
“We have now been able to show that treatment with THC has a tissue-dependent and dual effect on mTOR signaling and the metabolome,” explained Andras Bilkei-Gorzo from the Institute of Molecular Psychiatry at the UKB, who is also a researcher at the University of Bonn. “We concluded that long-term THC treatment initially has a cognition-enhancing effect by increasing energy and synaptic protein production in the brain, followed by an anti-aging effect by decreasing mTOR activity and metabolic processes in the periphery. Our study suggests that a dual effect on mTOR activity and the metabolome could be the basis for an effective anti-aging and cognition-enhancing drug.”
While the findings are promising, the study does have some limitations. The research was conducted in mice, and while these animals are commonly used as models for human biology, there are significant differences between species. It is not yet clear whether the same effects would be observed in humans, and further studies are needed to explore the potential therapeutic applications of THC in aging populations.
Additionally, the study focused on a specific dose and duration of THC treatment. It is possible that different doses or longer treatment periods could produce different results, either more beneficial or potentially harmful. Future research will need to explore these variables in greater detail to determine the optimal treatment protocol for maximizing the anti-aging effects while minimizing any potential risks.
Another important consideration is the broader impact of long-term THC use. Although the study did not find evidence of CB1 receptor downregulation—a common concern with prolonged cannabinoid exposure—the long-term effects on other physiological systems, particularly in older adults, remain to be fully understood.
In a previous study, researchers from Bonn, in collaboration with a team from the Hebrew University of Jerusalem, demonstrated that long-term, low-dose THC administration can reverse age-related decline in the brain by enhancing cognitive abilities and increasing synapse density in older mice. However, the question of whether these beneficial effects on the aging brain are connected to changes in mTOR signaling and metabolic processes remains unresolved.
The study, “Bidirectional Effect of Long-Term Δ9-Tetrahydrocannabinol Treatment on mTOR Activity and Metabolome,” was authored by Andras Bilkei-Gorzo, Britta Schurmann, Marion Schneider, Michael Kraemer, Prakash Nidadavolu, Eva C. Beins, Christa E. Müller, Mona Dvir-Ginzberg, and Andreas Zimmer.